Description
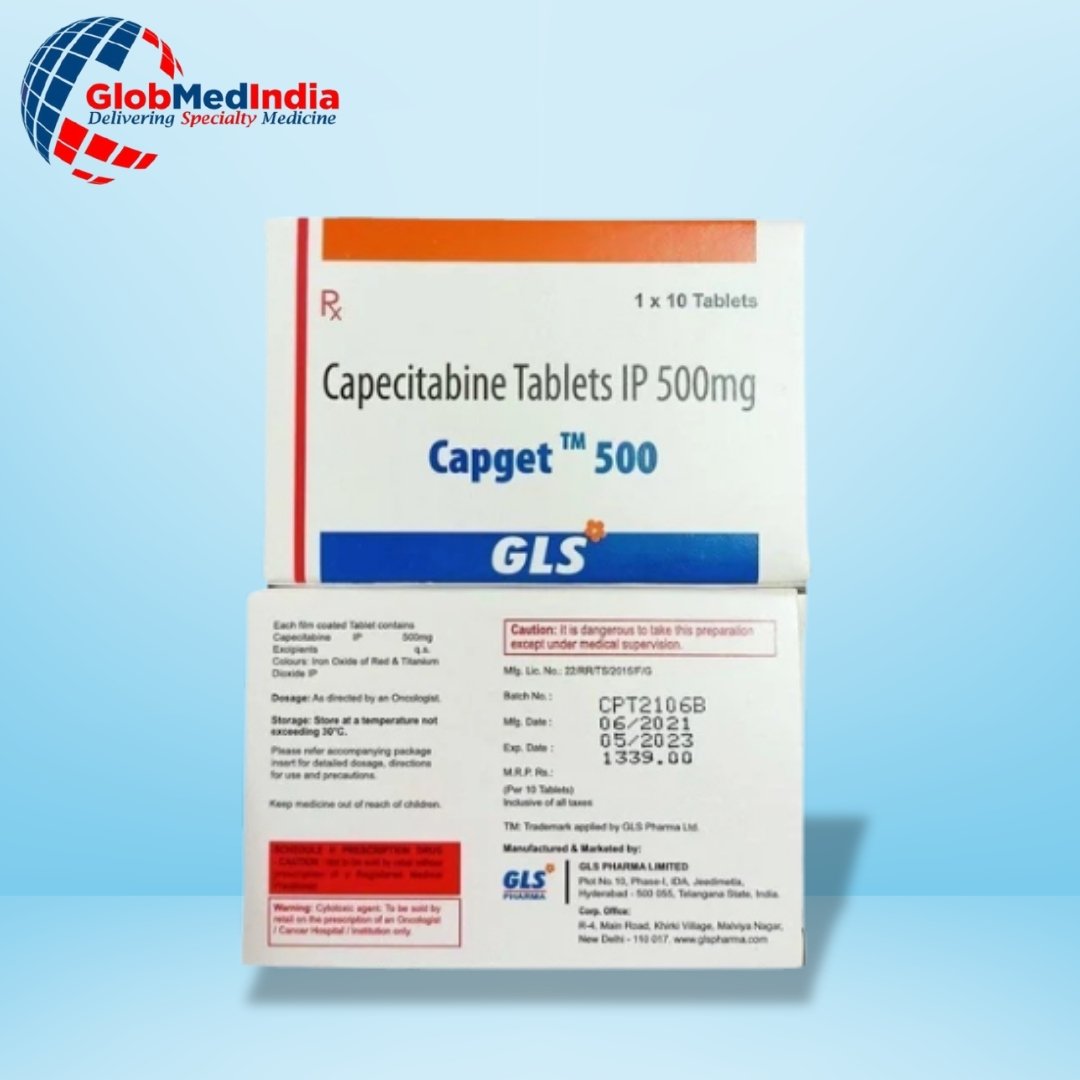
Capget 500mg Tablet contains the active ingredient Capecitabine. It belongs to the category of medicines known as antimetabolites. This medication is used in combination with other anticancer drugs for the treatment of Metastatic colorectal cancer and breast cancer. Cancer is a disease in which cells multiply in an abnormal and uncontrolled manner. It is also used for the prevention of new occurrences of cancer in the colon after the complete removal of the tumor by surgery.
The common side effects that are likely to occur with this medicine are loss of appetite, headache, runny nose, fever, hair loss, and dry mouth. During the treatment, your doctor may periodically monitor your complete blood counts, prothrombin time, INR, to prevent serious complications. Report to your doctor if you have been diagnosed with stomach ulcers, cataract, lung disease, problems in your heart, liver, skin, kidneys. Tell your physician before you undergo eye surgery or dental procedures. Inform your doctor if you have experienced any allergic or unusual reactions after taking this tablet. This medicine can cause fertility issues, inform your doctor about any fertility concerns you may have before starting treatment with this medicine.
Capget 500mg Tablet is not recommended for children and adolescents. Before taking this medicine, ask for physician advice if you are intolerant to sugars as this chemotherapy medication contains lactose. This drug will not be given if you have low white blood cell or platelet count, chickenpox, shingles, or enzyme DPD deficiency (dihydropyrimidine dehydrogenase). Take this medicine within thirty minutes after finishing a meal. This drug can cause fetal harm when administered to a pregnant woman.
We have strong global networks in AFRICA, Europe, United Kingdom, United States, Chile, Peru, Ethiopia, Romania, China, Japan, Russia, Ukraine, Dubai, Unites Arab Emirates, Thailand, Vietnam, Middle East and South East Countries.
Explore more products anti export













Reviews
There are no reviews yet.